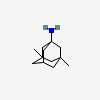Run the default task to build and export all views to CSV (tarball).
$ export PGHOST=127.0.0.1
$ export PGPORT=5432
$ makeRetrospective study of adamantane derivatives as neuroprotectants post-CVA.
mGluR5-Ca2+ apoptosis
Hippocampal long-tem potentiation
Rammes, G et al. Pharmacodynamics of memantine: an update. Current neuropharmacology vol. 6,1 (2008): 55-78. doi:157015908783769671
- Low-affinity voltage-dependent non-competitive antagonist at glutamatergic NMDA receptors
- NMDAR antagonist with higher affinity than Mg++, ultimatly inhibits prolonged Ca++ influx
- Non-competitive antagonist at 5-HT3 receptor, potency similar to that for the NMDA receptor
- Non-competitive antagonist at nAChRs with potencies possibly similar to NMDA and 5-HT3 receptors
- Agonist at D2 receptor with equal or slightly higher affinity than NMDA receptors
- Agonist at Sigma-1 receptor with low Ki ~= 2.6 μM (2600 nM)
- Therapeutic concentrations of memantine are most likely too low to have any sigmaergic effect, as a typical therapeutic dose is 20mg.
- Excessive doses of memantine (e.g. recreational use) may indeed activate Sigma-1
Drugs@FDA: Namenda® Tablets/Oral Solution (memantine hydrochloride)
Memantine is well absorbed after oral administration and has linear pharmacokinetics over the therapeutic dose range. It is excreted predominantly in the urine, unchanged, and has a terminal elimination half life of about 60-80 hours.
Following oral administration memantine is highly absorbed with peak concentrations reached in about 3-7 hours. Food has no effect on the absorption of memantine. The mean volume of distribution of memantine is 9-11 L/kg and the plasma protein binding is low (45%).
Majority (57-82%) of administered dose is excreted unchanged in urine; the remainder is converted primarily to three polar metabolites:
- N-gludantan conjugate
- 6-hydroxy memantine
- 1-nitroso-deaminated memantine
These metabolites possess minimal NMDA receptor antagonist activity. Memantine has a terminal elimination half-life of about 60-80 hours. Renal clearance involves active tubular secretion moderated by pH dependent tubular reabsorption.
Micuda S, Mundlova L, Anzenbacherova E, Anzenbacher P, Chladek J, Fuksa L, Martinkova J: Inhibitory effects of memantine on human cytochrome P450 activities: prediction of in vivo drug interactions. Eur J Clin Pharmacol. 2004 Oct;60(8):583-9. doi: 10.1007/s00228-004-0825-1. Epub 2004 Sep 16. [PubMed:15378224]
A cytochrome P450 monooxygenase (CYP2B6) involved in the metabolism of endocannabinoids and steroids (PubMed:21289075, PubMed:12865317). Mechanistically, uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (NADPH--hemoprotein reductase). Catalyzes the epoxidation of double bonds of arachidonoylethanolamide (anandamide) to 8,9-, 11,12-, and 14,15-epoxyeicosatrienoic acid ethanolamides (EpETrE-EAs), potentially modulating endocannabinoid system signaling (PubMed:21289075). Hydroxylates steroid hormones, including testosterone at C-16 and estrogens at C-2 (PubMed:21289075, PubMed:12865317). Plays a role in the oxidative metabolism of xenobiotics, including plant lipids and drugs (PubMed:11695850, PubMed:22909231). Acts as a 1,4-cineole 2-exo-monooxygenase (PubMed:11695850).
- Statins
- ACEi/ARB
- Ca++ channel blockers
- Alpha-2 antagonists (precedex, clonidine)
- Octeotide
- Epoprostenol
Dugbank DB01043
Human Metabolome Database HMDB0015177
KEGG Drug D08174
KEGG Compound C13736
PubChem Compound 4054
PubChem Substance 46506702
ChemSpider 3914
BindingDB 50062599
RxNav 6719
ChEBI 64312
ChEMBL CHEMBL807
Therapeutic Targets Database DAP000493
PharmGKB PA10364
-
5-hydroxytryptamine receptor 3A
- Gene Name: HTR3A
- Uniprot ID: P46098
- General Function
- Voltage-gated potassium channel activity
- Specific Function
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. This receptor is a ligand-gate...
-
Alpha-7 nicotinic cholinergic receptor subunit
-
Dopamine D2 receptor Gene Name: DRD2 Uniprot ID: P14416 General Function - Potassium channel regulator activity Specific Function - Dopamine receptor whose activity is mediated by G proteins which inhibit adenylyl cyclase.
-
Glutamate receptor ionotropic, NMDA 1
- Gene Name: GRIN1
- Uniprot ID: Q05586
- General Function
- Voltage-gated cation channel activity
- Specific Function
- NMDA receptor subtype of glutamate-gated ion channels with high calcium permeability and voltage-dependent sensitivity to magnesium. Mediated by glycine. This protein plays a key role in synaptic p...
-
Glutamate (NMDA) receptor (Protein Group)
- General Function
- Voltage-gated cation channel activity
- Specific Function
- NMDA receptor subtype of glutamate-gated ion channels with high calcium permeability and voltage-dependent sensitivity to magnesium. Mediated by glycine. This protein plays a key role in synaptic p...
- General Function
Components:
- Glutamate receptor ionotropic, NMDA 1: [Q05586](http://www.uniprot.org/uniprot/Q05586)
- Glutamate receptor ionotropic, NMDA 2A: [Q12879](http://www.uniprot.org/uniprot/Q12879)
- Glutamate receptor ionotropic, NMDA 2B: [Q13224](http://www.uniprot.org/uniprot/Q13224)
- Glutamate receptor ionotropic, NMDA 2C: [Q14957](http://www.uniprot.org/uniprot/Q14957)
- Glutamate receptor ionotropic, NMDA 2D: [O15399](http://www.uniprot.org/uniprot/O15399)
- Glutamate receptor ionotropic, NMDA 3A: [Q8TCU5](http://www.uniprot.org/uniprot/Q8TCU5)
- Glutamate receptor ionotropic, NMDA 3B: [O60391](http://www.uniprot.org/uniprot/O60391)
- GABA(A) Receptor (Protein Group)
- Curator comments
- The binding of memantine to this target is believed to be low to negligible.
- General Function
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine...
- Curator comments
Components
- Gamma-aminobutyric acid receptor subunit alpha-1 P14867
- Gamma-aminobutyric acid receptor subunit alpha-2 P47869
- Gamma-aminobutyric acid receptor subunit alpha-3 P34903
- Gamma-aminobutyric acid receptor subunit alpha-4 P48169
- Gamma-aminobutyric acid receptor subunit alpha-5 P31644
- Gamma-aminobutyric acid receptor subunit alpha-6 Q16445
- Gamma-aminobutyric acid receptor subunit beta-1 P18505
- Gamma-aminobutyric acid receptor subunit beta-2 P47870
- Gamma-aminobutyric acid receptor subunit beta-3 P28472
- Gamma-aminobutyric acid receptor subunit delta O14764
- Gamma-aminobutyric acid receptor subunit epsilon P78334
- Gamma-aminobutyric acid receptor subunit gamma-1 Q8N1C3
- Gamma-aminobutyric acid receptor subunit gamma-2 P18507
- Gamma-aminobutyric acid receptor subunit gamma-3 Q99928
- Gamma-aminobutyric acid receptor subunit pi O00591
- Gamma-aminobutyric acid receptor subunit theta Q9UN88
- Glycine receptors (Protein Group)
- General Function
- Transmitter-gated ion channel activity
- Specific Function
- The glycine receptor is a neurotransmitter-gated ion channel. Binding of glycine to its receptor increases the chloride conductance and thus produces hyperpolarization (inhibition of neuronal firing).
- General Function
Components
- Glycine receptor subunit alpha-1
- UniProt ID: P23415
- Glycine receptor subunit alpha-2
- UniProt ID: P23416
- Glycine receptor subunit alpha-3
- UniProt ID: O75311
- Glycine receptor subunit alpha-4
- UniProt ID: Q5JXX5
- Glycine receptor subunit beta
- UniProt ID: P48167
- Cytochrome P450 2B6
- Cytochrome P450 2A6
- Cytochrome P450 2C19
-
Solute carrier family 22 member 2 (putative)
- Gene Name: SLC22A2
- Uniprot ID: O15244
- General Function
- Quaternary ammonium group transmembrane transporter activity
- Specific Function
- Mediates tubular uptake of organic compounds from circulation.
- Mediates the influx of agmatine, dopamine, noradrenaline (norepinephrine), serotonin, choline, famotidine, ranitidine, histamin, creat...
-
Sodium/hydrogen exchanger 1
- Gene Name: SLC9A1
- Uniprot ID: P19634
- General Function
- Solute/proton antiporter activity
- Specific Function
- Involved in pH regulation to eliminate acids generated by active metabolism or to counter adverse environmental conditions. Major proton extruding system driven by the inward sodium ion chemical gr...
-
Solute carrier family 22 member 4
-
Multidrug and toxin extrusion protein 1
- Gene Name: SLC47A1
- Uniprot ID: Q96FL8
- General Function
- Monovalent cation/proton antiporter activity
- Specific Function
- Solute transporter for tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, N-methylnicotinamide (NMN), metformin, creatinine, guanidine, procainamide, topotecan, estrone sulfat...
References
Rammes, G., Danysz, W., & Parsons, C. G. (2008). Pharmacodynamics of memantine: an update. Current neuropharmacology, 6(1), 55–78. PubMed:19305788. doi:157015908783769671
Xia P, Chen HS, Zhang D, Lipton SA: Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010 Aug 18;30(33):11246-50. doi: 10.1523/JNEUROSCI.2488-10.2010. PubMed:20720132
Limapichat W, Yu WY, Branigan E, Lester HA, Dougherty DA: Key binding interactions for memantine in the NMDA receptor. ACS Chem Neurosci. 2013 Feb 20;4(2):255-60. doi: 10.1021/cn300180a. Epub 2012 Dec 7. PubMed:23421676
Muller F, Weitz D, Derdau V, Sandvoss M, Mertsch K, Konig J, Fromm MF: Contribution of MATE1 to Renal Secretion of the NMDA > Receptor Antagonist Memantine. Mol Pharm. 2017 Sep 5;14(9):2991-2998. doi: 10.1021/acs.molpharmaceut.7b00179. Epub 2017 Aug 2. PubMed:28708400
Molinaro G, Battaglia G, Riozzi B, Di Menna L, Rampello L, Bruno V, Nicoletti F: Memantine treatment reduces the expression of the K(+)/Cl(-) cotransporter KCC2 in the hippocampus and cerebral cortex, and attenuates behavioural responses mediated by GABA (A) receptor activation in mice. Brain Res. 2009 Apr 10;1265:75-9. doi: 10.1016/j.brainres.2009.02.016. Epub 2009 Feb 21. PubMed:19236854
Robinson DM, Keating GM: Memantine: a review of its use in Alzheimer's disease. Drugs. 2006;66(11):1515-34. [PubMed:16906789]
Cacabelos R, Takeda M, Winblad B: The glutamatergic system and neurodegeneration in dementia: preventive strategies in Alzheimer's disease. Int J Geriatr Psychiatry. 1999 Jan;14(1):3-47. PubMed:10029935
Rogawski MA, Wenk GL: The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease. CNS Drug Rev. 2003 Fall;9(3):275-308. PubMed:14530799
Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsons CG: The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Lett. 2001 Jun 22;306(1-2):81-4. PubMed:11403963
Nisijima K, Shioda K, Yoshino T, Takano K, Kato S: Memantine, an NMDA antagonist, prevents the development of hyperthermia in an animal model for serotonin syndrome. Pharmacopsychiatry. 2004 Mar;37(2):57-62. doi: 10.1055/s-2004-815526. PubMed:15048612
Aracava Y, Pereira EF, Maelicke A, Albuquerque EX: Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther. 2005 Mar;312(3):1195-205. Epub 2004 Nov 2. PubMed:15522999
Maskell PD, Speder P, Newberry NR, Bermudez I: Inhibition of human alpha 7 nicotinic acetylcholine receptors by open channel > blockers of N-methyl-D-aspartate receptors. Br J Pharmacol. 2003 Dec;140(7):1313-9. doi: 10.1038/sj.bjp.0705559. PubMed:14645141
Pohanka M: Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int J Mol Sci. 2012;13(2) :2219-38. doi: 10.3390/ijms13022219. Epub 2012 Feb 17. PubMed:22408449
Seeman P, Caruso C, Lasaga M: Memantine agonist action at dopamine D2High receptors. Synapse. 2008 Feb;62(2):149-53. PubMed:18000814
Serra G, Demontis F, Serra F, De Chiara L, Spoto A, Girardi P, Vidotto G, Serra G: Memantine: New prospective in bipolar disorder treatment. World J Psychiatry. 2014 Dec 22;4(4):80-90. doi: 10.5498/wjp.v4.i4.80. [PubMed:25540723](http://www.ncbi. nlm.nih.gov/pubmed/25540723)
Nakaya K, Nakagawasai O, Arai Y, Onogi H, Sato A, Niijima F, Tan-No K, Tadano T: Pharmacological characterizations of memantine-induced disruption of prepulse inhibition of the acoustic startle response in mice: involvement of dopamine D2 and 5-HT2A receptors. Behav Brain Res. 2011 Mar 17;218(1):165-73. doi: 10.1016/j.bbr.2010.11.053. Epub 2010 Dec 3. PubMed:21130810
Mancini M, Ghiglieri V, Bagetta V, Pendolino V, Vannelli A, Cacace F, Mineo D, Calabresi P, Picconi B: Memantine alters striatal plasticity inducing a shift of synaptic responses toward long-term depression. Neuropharmacology. 2016 Feb; 101:341-50. doi: 10.1016/j.neuropharm.2015.10.015. Epub 2015 Dec 3. PubMed:26471421
Kotermanski SE, Wood JT, Johnson JW: Memantine binding to a superficial site on NMDA receptors contributes to partial trapping. J Physiol. 2009 Oct 1;587(Pt 19):4589-604. doi: 10.1113/jphysiol.2009.176297. Epub 2009 Aug 17. [PubMed:19687120] (http://www.ncbi.nlm.nih.gov/pubmed/19687120)
Cacabelos R, Takeda M, Winblad B: The glutamatergic system and neurodegeneration in dementia: preventive strategies in Alzheimer's disease. Int J Geriatr Psychiatry. 1999 Jan;14(1):3-47. PubMed:10029935
Rogawski MA, Wenk GL: The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease. CNS Drug Rev. 2003 Fall;9(3):275-308. PubMed:14530799
Robinson DM, Keating GM: Memantine: a review of its use in Alzheimer's disease. Drugs. 2006;66(11):1515-34. PubMed:16906789
Rogawski MA: Low affinity channel blocking (uncompetitive) NMDA receptor antagonists as therapeutic agents--toward an understanding of their favorable tolerability. Amino Acids. 2000;19(1):133-49. PubMed:11026482
Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsons CG: The N-methyl-D-aspartate receptor channel blockers memantine, > MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems > in a non-competitive manner. Neurosci Lett. 2001 Jun 22;306(1-2):81-4. PubMed:11403963
Kishi T, Matsunaga S, Oya K, Nomura I, Ikuta T, Iwata N: Memantine for Alzheimer's Disease: An Updated Systematic Review and Meta-analysis. J Alzheimers Dis. 2017;60(2):401-425. doi: 10.3233/JAD-170424. PubMed:28922160
Brianne Kuns; Dona Varghese (2019). StatPearls: Memantine. StatPearls Publishing. 135 million people will live with dementia worldwide by 2050 Link
Sandoz Monograph: Memantine hydrochloride oral tablets Link
Alzheimer's disease international: Global Impact of Dementia 2013 File
Memantine Product File

![Adamantane [PubChem CID: 9238]](https://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=9238)